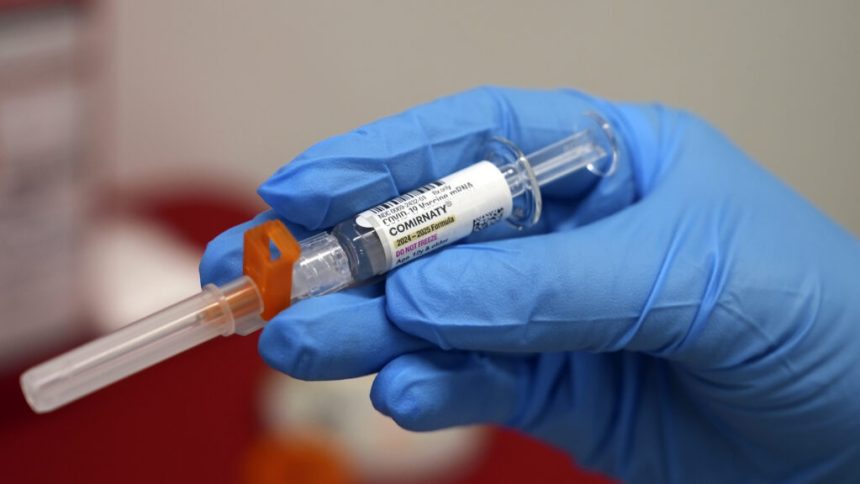
WASHINGTON — A key federal health authority has approved new guidance allowing anyone aged 6 months and older to receive an updated Covid-19 vaccine following a discussion of the associated risks and benefits with their healthcare provider. While this new guidance retains insurance coverage for individuals wishing to get vaccinated, it remains unclear whether a prescription will still be necessary for some people to obtain the shot, as permitted by the Food and Drug Administration in August.
Jim O’Neill, the acting director of the Centers for Disease Control and Prevention, confirmed on Monday that he approved the recommendations put forth by an advisory panel to the agency over two weeks prior. Additionally, he endorsed a suggestion for children under 4 years of age to receive the measles, mumps, and rubella vaccine separately from the chickenpox vaccine, rather than as a combined shot.
“Informed consent is making a comeback,” O’Neill stated. “The CDC’s blanket recommendation for ongoing COVID-19 boosters in 2022 discouraged healthcare professionals from discussing the personal risks and benefits of the vaccine with patients or their parents. That dynamic has shifted today.”
These approvals enable states to request updated Covid vaccines for low-income children eligible through the Vaccines for Children program. The delay in signing off on these recommendations meant that this year, children under the program were unable to access them.
“This creates a two-tiered system regarding access to Covid vaccinations for families who desire them,” remarked Jason Terk, a pediatrician based in North Texas, before the recommendations were finalized. “It appears that those currently in power within our national public health framework aim to erode public confidence in Covid-19 vaccinations and restrict access. It’s disappointing, especially given that children are at a heightened risk for complications from Covid-19.”
While the recommendations were initially issued over two weeks ago, O’Neill’s endorsement was crucial for enabling the shipment of doses to states. The absence of this initial approval resulted in approximately 50% of U.S. children depending on the VFC program being unable to access the updated Covid vaccines, according to immunization program experts.
A revised schedule for routine childhood vaccinations is also anticipated to be released on Tuesday, representing some of the first adjustments made by the Trump administration to the overall vaccine schedule since pledging to reform how vaccines are reviewed, approved, and recommended in the U.S.


![Nina Chanel Abney and Jeffrey Deitch On Finding the True Artist’s Voice [Exclusive] Nina Chanel Abney and Jeffrey Deitch On Finding the True Artist’s Voice [Exclusive]](https://americanfocus.online/wp-content/uploads/2025/10/abney-2-150x150.jpg)

