Growing Synthetic Brain Tissue in the Lab: A Breakthrough in Neuroscience Research
Studying real, living, three-dimensional brain tissue for research purposes has always been challenging due to the ethical implications of obtaining such tissue. However, scientists have made significant progress in creating realistic brain tissue models in the laboratory for experimentation.
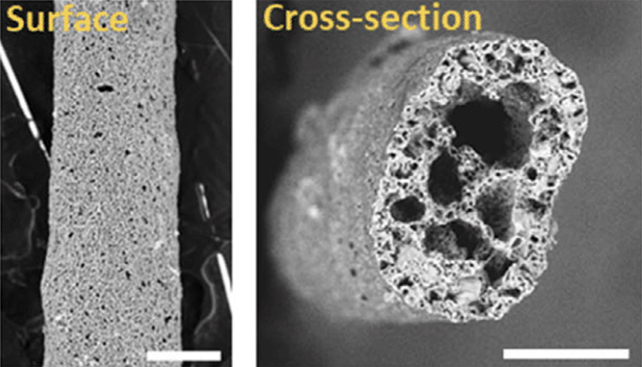
A team of researchers at the University of California, Riverside (UCR) has developed a groundbreaking technique involving a tiny scaffolding just 2 millimeters wide, onto which neural stem cells can be attached and mature into fully functional neurons.
This scaffolding, known as BIPORES (Bijel-Integrated PORous Engineered System), is primarily made of polyethylene glycol (PEG), a common polymer. The researchers engineered the PEG to be ‘sticky’ for brain cells, eliminating the need for conventional coatings that could affect the reliability of scientific results.
The addition of silica nanoparticles and the unique shape of the PEG create a matrix of porous structures that mimic a sponge, providing a conducive environment for cell adhesion and growth. The curved and stabilized structure promotes natural cell growth and organization, resulting in brain-like clusters.
Iman Noshadi, a bioengineer at UCR, explains, “The material ensures cells get what they need to grow, organize, and communicate with each other in brain-like clusters. Because the structure more closely mimics biology, we can start to design tissue models with much finer control over how cells behave.”
This innovative approach overcomes many challenges associated with traditional methods of growing brain tissue in the lab. The researchers anticipate that this technique will produce tissue that closely resembles human brain tissue, is more stable, and can mature more effectively than current models without the use of foreign chemicals or animal-derived materials.
Prince David Okoro, another bioengineer at UCR, notes, “Since the engineered scaffold is stable, it permits longer-term studies. That’s especially important as mature brain cells are more reflective of real tissue function when investigating relevant diseases or traumas.”
Furthermore, by utilizing neural stem cells derived from human blood or skin cells, researchers have the potential to create personalized ‘test neurons’ tailored to individual patients. This personalized approach could be vital in advancing research on neurodegenerative diseases, strokes, and other brain injuries.
By reducing dependence on animal brain testing and producing brain tissue models that closely resemble human tissue, researchers can enhance the relevance and ethical standards of their studies. This breakthrough not only benefits scientific research but also opens up new possibilities for personalized medicine.
While there are still challenges to overcome, such as scaling up the technique for larger tissue models, the researchers are optimistic about its potential applications beyond the brain. They believe that this approach could be extended to study other organs in the body, such as the liver.
Iman Noshadi concludes, “An interconnected system would let us see how different tissues respond to the same treatment and how a problem in one organ may influence another. It is a step toward understanding human biology and disease in a more integrated way.”
The research findings have been published in Advanced Functional Materials.