Understanding the Connection Between Aging, DNA Methylation, and Colorectal Cancer Risk
As we age, the chemical marks on our DNA undergo a gradual shift. A recent study has uncovered that this phenomenon, known as Aging and Colon Cancer-Associated (ACCA) drift, is driven by inflammation and disrupted cell signaling in gut stem cells. This discovery sheds light on why the risk of colorectal cancer increases with age.
The research, conducted by an international team of scientists, reveals that ACCA drift involves alterations in DNA methylation, which can activate or deactivate genes without changing the DNA sequence. In this case, the drift leads to the progressive silencing of genes that play a role in suppressing tumor formation, paving the way for an accumulation of cancer risk in the gut.
According to molecular biologist Francesco Neri from the University of Turin, Italy, “We observe an epigenetic pattern that becomes increasingly apparent with age.” The study builds upon existing knowledge linking epigenetic drift to cancer and the age-related increase in colorectal cancer risk.
The researchers analyzed tissue samples from healthy human colons and colon cancer tumors to identify common methylation patterns. They found similar gene silencing patterns in older individuals and cancerous tissue, suggesting a shared underlying process.
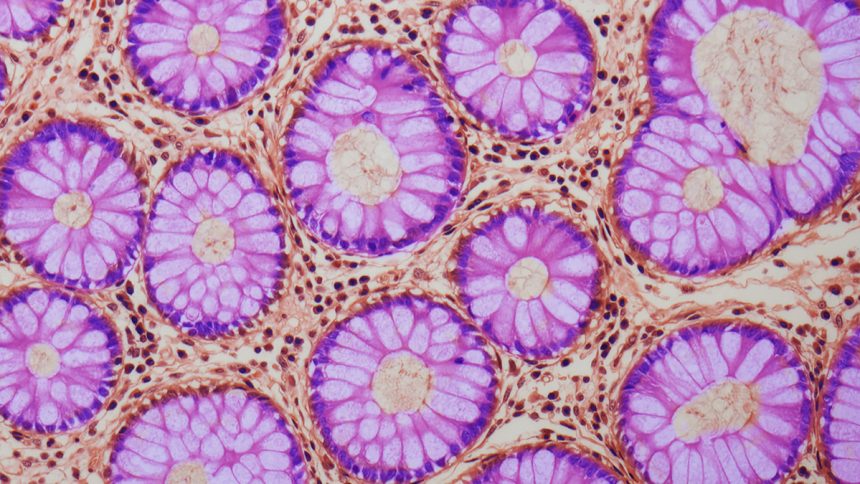
Further investigations using mouse models and organoids revealed that ACCA drift originates in intestinal crypts, which are small structures in the gut lining that house stem cells responsible for renewing the intestinal lining. The drift begins within these stem cells and spreads as the crypts divide and expand.
The study focused on how inflammation, reduced growth signaling, and iron imbalance in intestinal crypt stem cells disrupt methylation processes, leading to gene deactivation and potentially facilitating cancer development. Molecular biologist Anna Krepelova explains, “Over time, more and more areas with an older epigenetic profile develop in the tissue. Through the natural process of crypt division, these regions continuously enlarge and can continue to grow over many years.”
As stem cell-driven crypts multiply, regions of tissue with an aged, cancer-prone epigenetic profile expand, creating more vulnerable areas throughout the gut over time. Inflammation, iron imbalance, and decreased growth signaling can accelerate epigenetic drift, hastening the aging process and increasing susceptibility to cancer.
However, the study offers a glimmer of hope by demonstrating that it is possible to slow and partially reverse epigenetic drift in organoids by enhancing iron uptake or restoring specific cell growth signals. Krepelova remarks, “For the first time, we are seeing that it is possible to tweak the parameters of aging that lie deep within the molecular core of the cell.”
The findings, published in Nature Aging, provide valuable insights into the complex interplay between aging, DNA methylation, and colorectal cancer risk. Understanding these mechanisms opens up new possibilities for targeted interventions to mitigate age-related cancer vulnerabilities.