Unlocking the Secrets of Fat Cell Formation: A Breakthrough Study
Understanding the process by which cells transform into fat cells is crucial in addressing health conditions such as obesity and type 2 diabetes. A recent study has provided valuable insights into how we can potentially prevent cells from becoming fat cells.
A team of researchers from the Korea Advanced Institute of Science and Technology delved into the role of the PPARγ protein, described as a “master regulator” of fat cell formation. When activated, PPARγ initiates a cascade of genetic instructions that drive a cell to become a fat cell, also known as an adipocyte.
Through their analysis of mouse cells and models, the scientists identified a specific epigenetic switch that can block the fat-producing signals of PPARγ. This switch, which controls gene behavior without altering DNA, plays a crucial role in regulating adipocyte differentiation.
Molecular biologist Dae-Sik Lim emphasized the significance of this discovery, stating that it sheds light on how adipocyte identity changes are controlled at an epigenetic level.
The study focused on two proteins, YAP and TAZ, which are part of the Hippo signaling pathway. This pathway plays a vital role in determining cell fate, including whether a cell will become a fat cell. Previous research hinted at the involvement of YAP and TAZ in inhibiting fat cell formation, but the exact mechanism was unclear until now.
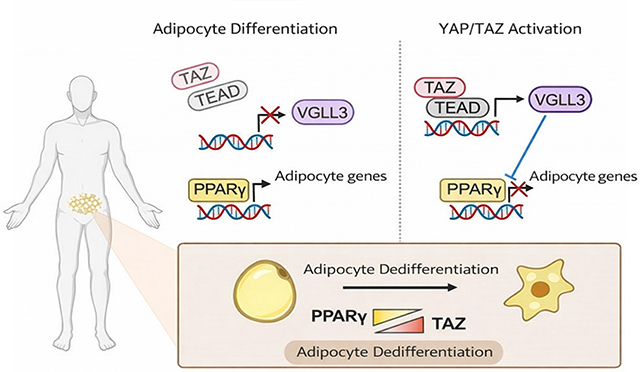
The researchers demonstrated that YAP and TAZ can interfere with the fat cell activation genes targeted by PPARγ, effectively halting the process of fat cell formation. The Hippo signaling pathway acts as a regulatory mechanism for YAP and TAZ, ensuring precise control over their activity.
By manipulating the Hippo signaling pathway in mice, the researchers observed a reversal in the developmental path of existing fat cells. Instead of fully reverting to stem cells, these fat cells lost some of their defining features and exhibited characteristics of precursor cells.
The findings of this study provide valuable insights into the mechanisms underlying fat cell production and regulation. While the research was conducted in mice, the implications for human health are significant, considering the link between excess fat accumulation and various metabolic diseases.
Developing a deeper understanding of how PPARγ influences the formation of fat cells could pave the way for novel treatment strategies for metabolic disorders. Targeting fat accumulation with greater precision may offer new avenues for personalized therapies in the future.
Lim expressed optimism about the study’s implications for advancing personalized treatment approaches for patients with metabolic conditions. The research has been published in Science Advances and marks a significant step forward in unraveling the complexities of fat cell biology.