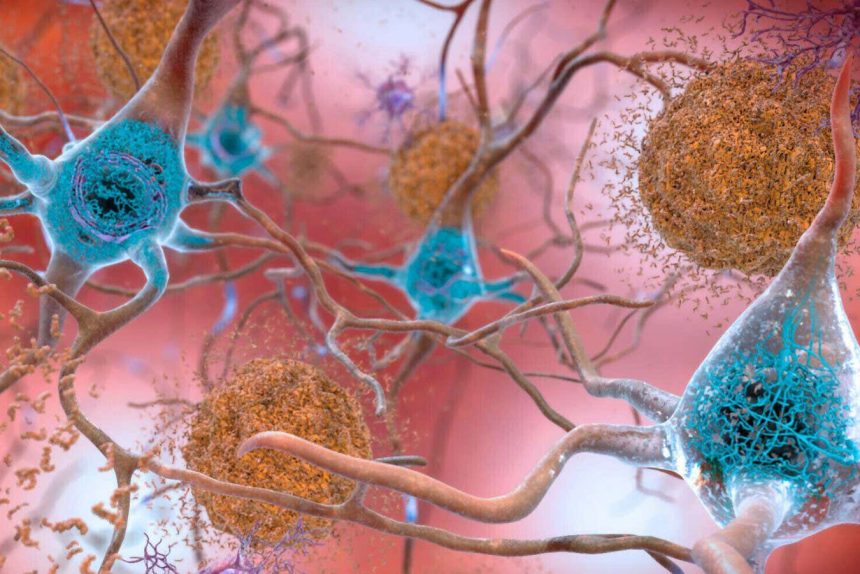
This increased activity may help the microglia clear away tau tangles more efficiently, preventing them from accumulating and causing cognitive decline, says Prater. “It could be that you’re preventing the spread of tau pathology through the brain,” she says. The team also found that the resilient individuals had fewer tau tangles in their dorsolateral prefrontal cortex compared with those with Alzheimer’s, which supports this idea.
Both studies highlight the complex interplay between amyloid plaques, tau tangles, and immune cells in the brain, shedding light on why some individuals remain resilient to Alzheimer’s disease despite having the pathological hallmarks of the condition. Understanding these mechanisms could pave the way for new therapeutic strategies that target tau accumulation and enhance the function of microglia to protect against cognitive decline.
Further research is needed to confirm these findings and explore the potential of these pathways as targets for future treatments. By unraveling the mysteries of Alzheimer’s resilience, scientists are uncovering new insights into the mechanisms underlying this devastating disease and offering hope for effective interventions in the future.
Recent studies have shed light on the genetic activity in resilient individuals compared to those with Alzheimer’s disease. One study, presented at a meeting of the Society for Neuroscience in San Diego, California, revealed that the genetic activity in resilient individuals was similar to that of individuals without Alzheimer’s disease. This suggests that certain processes, such as those related to cell health, may go awry in Alzheimer’s disease.
According to researcher Prater, the interruption of crucial processes in cells can have detrimental effects. In Alzheimer’s disease, microglia – immune cells in the brain – showed decreased activity in genes involved in metabolizing energy. This decreased activity was more pronounced in individuals with Alzheimer’s disease, indicating that microglia may be more inflammatory in this condition. This inflammation can disrupt connections between neurons and contribute to cell death.
The findings of these studies suggest that the human brain has mechanisms to mitigate tau burden, a protein associated with Alzheimer’s disease. Understanding how these mechanisms work could lead to new treatments that may prevent Alzheimer’s disease, rather than just slowing its progression. While there is still a long way to go before a therapeutic intervention is developed, the research indicates that there is hope and promise in the biology of Alzheimer’s disease resilience.
In conclusion, these studies provide valuable insights into the genetic activity in resilient individuals and how it differs from those with Alzheimer’s disease. By unraveling the mechanisms behind Alzheimer’s resilience, researchers may be able to develop more effective treatments for this devastating condition. The journey towards finding a cure for Alzheimer’s disease continues, fueled by the promising findings of these studies.