Revolutionizing Cancer Treatment with In-Body CAR T-Cell Therapy

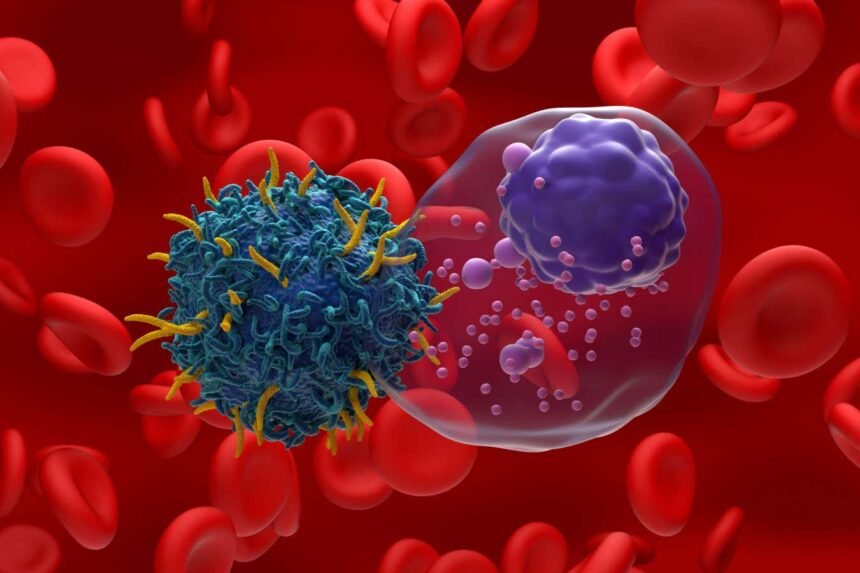
Illustration of CAR T-cell therapy targeting multiple myeloma, a type of blood cancer
Nemes Laszlo/Alamy
Advancements in cancer treatment have led to the development of CAR T-cell therapy, a groundbreaking approach that involves genetically engineering a patient’s immune cells to target and destroy cancer cells. While this therapy holds great promise, its current process is complex and costly. However, recent research has introduced a novel method of creating personalized CAR T-cell therapy within the body of non-human animals, offering a more streamlined and cost-effective solution.
Traditionally, CAR T-cell therapy is utilized in the treatment of certain types of blood cancers, such as leukaemia, by extracting T-cells from a patient’s blood, modifying them in a lab to target specific cancer cells, and reintroducing them into the body. This process, as effective as it may be, involves multiple steps and can be prohibitively expensive.
To address these challenges, researchers have developed a technique using RNA molecules encapsulated in fatty capsules to deliver instructions for producing proteins that can target cancer cells. This in-body approach results in temporary modification of T-cells, allowing them to recognize and eliminate cancer cells. Unlike traditional CAR T-cell therapy, this method does not require extensive laboratory manipulation and can be administered directly into the body.
In experimental studies involving mice and monkeys, this innovative approach demonstrated promising results. Mice injected with the fatty capsules showed a significant reduction in tumour cells, with some achieving complete elimination of cancer cells. Similarly, monkeys treated with the therapy experienced rapid clearance of cancerous cells without adverse effects, showcasing the potential effectiveness of in-body CAR T-cell therapy.
While this new approach presents a simpler and more cost-effective alternative to traditional CAR T-cell therapy, some limitations remain. The temporary nature of the modified T-cells may necessitate repeated treatments, and further research is required to assess long-term effectiveness and safety in human patients.
Lead researcher, Carl June, has already initiated human trials to evaluate the feasibility and efficacy of in-body CAR T-cell therapy. With ongoing advancements in this field, this innovative treatment approach could offer new hope for cancer patients seeking effective and accessible therapies.
Topics: Cancer Treatment, CAR T-Cell Therapy, Immunotherapy, Genetic Engineering