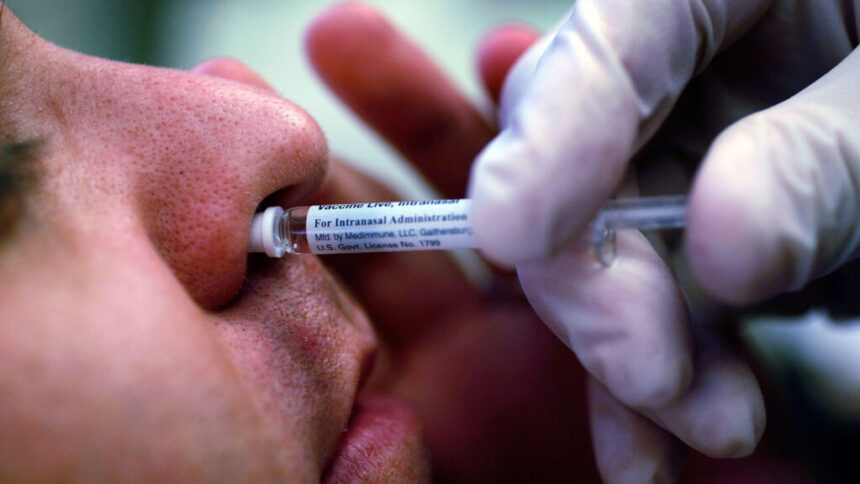
The Food and Drug Administration has recently granted approval to AstraZeneca for the home administration of its nasal spray influenza vaccine, FluMist. This means that starting in the fall of 2025, individuals will have the option to order the vaccine and administer it to themselves or their children at home.
FluMist is unique as it is the only flu vaccine that is administered through a nasal spray rather than an injection. It is approved for use in individuals aged 2 to 49. With this FDA approval, FluMist becomes the only flu vaccine in the United States that can be self-administered at home without the need for a healthcare professional.
Peter Marks, the director of the FDA’s Center for Biologics Evaluation and Research, expressed that this approval provides a new and convenient option for individuals and families to receive a safe and effective seasonal influenza vaccine. While FluMist will still be available in doctors’ offices and pharmacies for administration by healthcare providers, the new option allows for the vaccine to be ordered for home use by individuals aged 18 and older.
To order the vaccine for home administration, individuals can utilize an online portal called FluMist Home. They will need to fill out an online questionnaire, which will be reviewed by a pharmacist to determine eligibility for self-administration. Once approved, the vaccine will be delivered to their home.
Iskra Reic, AstraZeneca’s executive vice president for vaccines and immune therapies, stated that the approval of FluMist for self-administration marks a significant step in making vaccines more accessible in the fight against the annual burden of influenza.
It is important to note that some side effects may occur after receiving FluMist. These include fever over 100 degrees Fahrenheit in children aged 2 to 6, runny nose and nasal congestion in individuals aged 2 to 49, and a sore throat in adults aged 18 to 49.
Overall, the approval of FluMist for home administration provides a convenient and accessible option for individuals and families to receive their seasonal influenza vaccine. This development is expected to enhance flexibility and convenience in vaccine administration while continuing to prioritize safety and effectiveness in flu prevention.