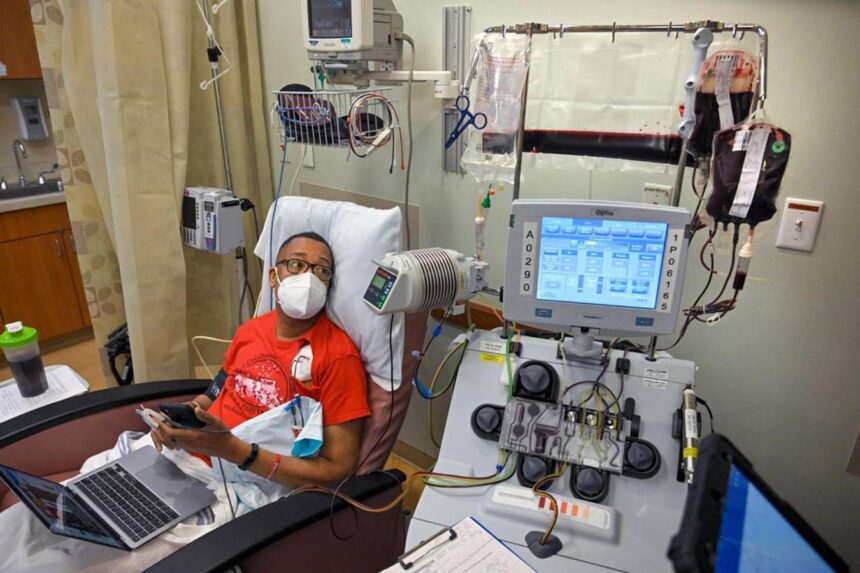
The Centers for Medicare and Medicaid Services recently announced a new program aimed at improving patient access to gene therapies for sickle cell disease. This initiative, supported by both the Biden and Trump administrations, will involve 33 states, the District of Columbia, and Puerto Rico. The program will focus on tying payment for gene therapies to positive clinical outcomes, making these treatments more accessible to patients. This model could potentially set a precedent for future agreements regarding cell and gene therapies.
Sickle cell disease (SCD) is a genetic blood disorder that affects millions of people globally, with approximately 100,000 individuals in the United States living with the condition. SCD causes severe pain, anemia, organ damage, and infections, reducing life expectancy by more than 20 years on average. The most common form of SCD is sickle cell anemia, characterized by abnormal hemoglobin molecules that distort red blood cells into a sickle shape.
Traditionally, treatments for SCD have included pain medications, antibiotics, and hydroxyurea to manage symptoms. However, recent advancements in gene therapy have led to the development of novel treatments like Lyfgenia and Casgevy, which show promising results in decreasing or eliminating pain crises in SCD patients. These gene therapies come with a high price tag, with initial launch prices of $2.2 million and $3.1 million, respectively.
The new program will allow Medicaid to negotiate outcomes-based agreements with manufacturers of gene therapies, ensuring reimbursement based on predefined clinical thresholds. Participating states represent a significant portion of Medicaid beneficiaries with SCD, potentially expanding access to transformative care for those in need. Additionally, federal support is available to assist states in implementing these agreements and tracking patient outcomes.
While access to gene therapies remains a challenge due to their high costs and regulatory hurdles, programs like this one aim to streamline the process and improve access for patients. By gathering evidence and coordinating contracts across state agencies, the program hopes to pave the way for wider access to gene therapies for various diseases. Overcoming barriers to patient access is essential for unlocking the full potential of gene therapies in improving health outcomes.
In conclusion, the new program for gene therapies targeting sickle cell disease represents a significant step towards enhancing patient access to transformative treatments. By leveraging outcomes-based agreements and federal support, states can work towards making these innovative therapies more accessible to those in need. This initiative could serve as a blueprint for improving access to other cell and gene therapies in the future.