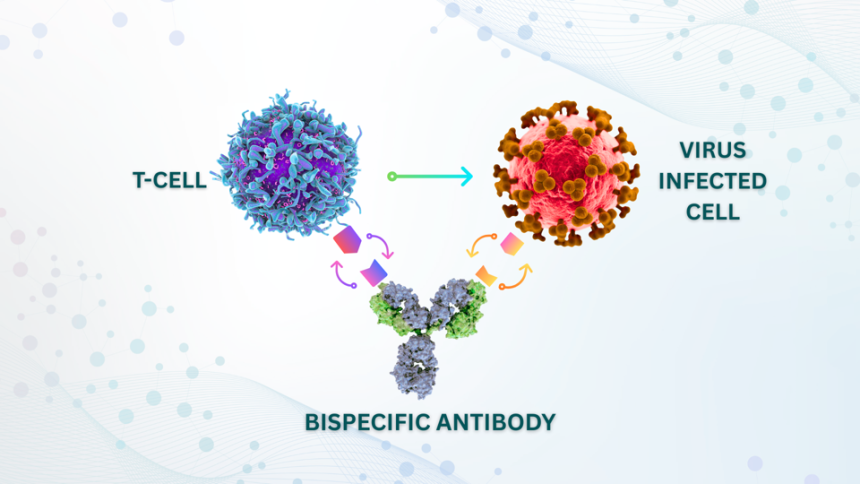
In this representation, one arm of the bispecific antibody binds to an infected cell displaying cytomegalovirus proteins while the other arm attaches to a T cell. This clever design acts like a bridge, drawing the T cell closer to the infected cell and activating its natural ability to kill.
ACCESS Health International
Almost everyone will come into contact with cytomegalovirus (CMV) during their lifetime. While it’s benign and dormant for many, it poses serious threats to vulnerable populations. Existing antiviral drugs provide only limited control over CMV and are often associated with significant side effects. A recent study published in Science Advances introduces a promising innovation: engineered bispecific antibodies designed to mobilize the immune system’s T cells to target and eradicate CMV-infected cells. This breakthrough holds the potential to revolutionize how we confront one of the globe’s most widespread yet underappreciated viral threats.
Understanding the Mechanism of Bispecific Antibodies
The human immune system is specially structured to recognize self from non-self entities, executing the elimination of pathogens like viruses and bacteria. T cells play a pivotal role in this immunological defense, identifying and destroying infected cells. In the case of cytomegalovirus, the immune response is often sufficient to contain the virus without completely eradicating it. This is where bispecific antibodies emerge as a game-changer.
Unlike traditional antibodies, which typically target a single pathogen, bispecific antibodies can simultaneously bind to two distinct targets. In this scenario, one arm of the antibody latches onto a CMV-infected cell, while the other arm connects directly to a T cell, functioning as a link that drives the T cell to engage and attack the infected cell.
Promising Results from Initial Trials for High-Risk Populations
The research featured in Science Advances explored the effectiveness of this bispecific strategy in laboratory settings. These antibodies successfully redirected T cells to seek out and destroy CMV-infected cells with remarkable precision. Notably, this process did not necessitate pre-engineering of specialized T cells, unlike existing cancer therapies such as CAR-T treatments. Instead, regular patient T cells were efficiently activated in real-time by these bispecific antibodies, thereby simplifying clinical applications.
Crucially, the bispecific antibodies did not indiscriminately activate T cells against healthy cells, a common risk in immune therapies. Controlled targeting minimizes the chances of collateral damage that could be as harmful as the virus itself. In addition to preliminary lab assessments, bispecific antibodies are demonstrating effectiveness in early-stage trials against cytomegalovirus, with numerous biotech companies initiating efforts to translate these laboratory findings into viable human therapies, particularly for those undergoing organ transplants.
Patients who undergo organ transplants are particularly at risk from CMV, with an alarming one-third developing severe complications due to this virus. The immunosuppressive medications that transplant recipients must take compromise their immune systems. If these bispecific antibodies can help manage CMV without significant toxic effects, it would represent a landmark advancement in patient care.
The Importance of This Innovation
What distinguishes this bispecific antibody strategy is its dependability and scalability. Unlike personalized immune therapies requiring extended processing times, bispecific antibodies can be manufactured en masse, stored, and dispensed in a manner akin to traditional antibody medications. This effective production model ensures that they can be quickly administered to patients in critical conditions. Furthermore, the antibody framework can potentially be repurposed to direct T cells against other persistent chronic infections, such as Epstein-Barr virus and various hepatitis viruses. There is even potential to tackle certain cancers, which also rely on evading immune detection.
Over recent years, immunotherapy has transformed cancer treatment by leveraging the body’s immune response to confront malignancies. However, similar approaches have rarely been applied to chronic viral diseases. The advent of bispecific antibodies for cytomegalovirus represents a paradigm shift—shifting from indefinite viral suppression through pharmacological means to empowering the immune system to function as it was designed to.
Addressing Challenges on the Horizon
Despite the promising prospects, the development of bispecific antibodies faces several significant challenges. The fabrication of these complex antibodies is considerably more intricate than traditional antibodies, requiring assurance of stability, safety, and consistent behavior in human subjects prior to any potential regulatory approvals. Additionally, long-term studies are necessary to verify that the precision in T cell killing remains effective over time.
Moreover, the cytomegalovirus is notoriously adept at evading immune responses, possessing multiple mechanisms to thwart immune action. Researchers are investigating whether a combined approach that integrates bispecific antibodies with vaccines or antiviral medications could offer the best defense against CMV.
Nonetheless, the early implications for clinical practice are thrilling. A therapy capable of diminishing CMV-related complications in transplant recipients could shorten hospital stays and lessen the reliance on expensive drug regimens. Above all, it has the potential to avert long-term organ rejection and save lives, addressing a substantial burden on healthcare systems tasked with managing CMV-related health issues.
Looking Ahead: The Future of Bispecific Antibodies
The journey toward the realization of bispecific antibody development is still in its nascent stages, but the direction is unmistakable. Progress in research is rapidly advancing from the laboratory to clinical environments, with human trials expected in the near future. If outcomes are favorable, this innovative approach could define new standards for managing persistent viral infections.
Science often develops in phases, at times in small increments and occasionally in significant leaps. This revolutionary breakthrough edges closer to monumental progress. By effectively connecting T cells to infected cells, bispecific antibodies may ultimately equip us with the means to outsmart viruses that have troubled humanity for centuries.