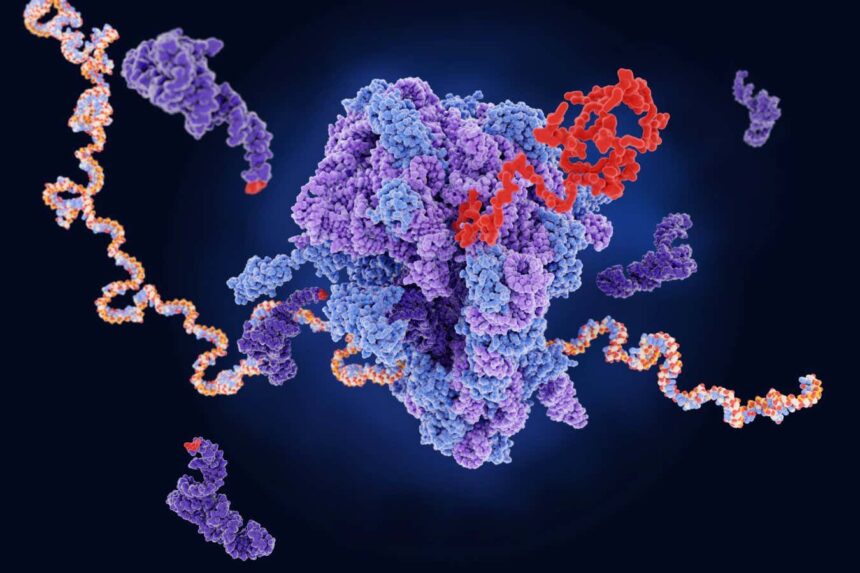
A ribosome (centre) producing a protein (red) from mRNA. The dark purple strands represent transfer RNA, which are also involved in protein production
Recent research has uncovered a potential key factor in cellular ageing that may have far-reaching implications for understanding the ageing process in cells. A study conducted on freshwater fish known as killifish has revealed that as these fish age, the ribosomes responsible for protein synthesis in their cells begin to stall while producing a specific class of proteins, setting off a detrimental cycle of decline.
According to Alessandro Cellerino from the Leibniz Institute on Aging in Germany, this discovery opens up new possibilities for addressing age-related cognitive decline. He suggests that the focus should be on enhancing cognition and preventing cognitive deterioration rather than merely extending lifespan.
The process of protein synthesis starts with the transcription of DNA into mRNA, which carries the instructions for protein production. The mRNA molecules are then translated by ribosomes, the cellular protein factories, into amino acids that form the proteins.
However, studies have shown that in ageing cells, the correlation between mRNA levels and protein production diminishes, leading to a decrease in protein synthesis despite adequate mRNA availability.
Cellerino and his team have delved into this phenomenon by investigating ribosomes in the ageing brains of killifish. By employing a novel technique, they were able to observe ribosomes stalling at specific codons, particularly those encoding the amino acids arginine and lysine, as the fish aged. This stalling hinders the completion of protein synthesis, particularly affecting proteins involved in DNA and RNA binding.
These DNA- and RNA-binding proteins play crucial roles in various cellular functions, such as RNA processing, DNA repair, and protein production. The ribosome stalling phenomenon appears to link multiple age-related cellular changes, including DNA damage, reduced RNA production, and impaired protein synthesis.
Moreover, the researchers suggest that a feedback loop may exacerbate the situation, as the stalled ribosomes lead to decreased production of ribosomes themselves, further hampering protein synthesis.
While the study focused on killifish, there is speculation about whether similar ribosomal stalling occurs in human brains. Recent research by Gene Yeo at the University of California San Diego has indicated alterations in RNA-binding proteins in ageing human neurons, hinting at a potential connection with Cellerino’s findings.
If validated in human studies, these findings could pave the way for novel treatments targeting age-related brain conditions. In killifish, ribosomal stalling triggers an inflammatory response, leading to chronic inflammation, a prominent factor in age-related brain deterioration. Experimental drugs that block this signaling pathway show promise in mitigating these conditions.
However, the implications for lifespan extension remain uncertain, as the mechanisms underlying ribosomal stalling and its universality across different organs are yet to be fully understood. Further research is needed to unravel the intricacies of this process and explore its potential therapeutic applications in combating age-related cognitive decline.