The Dark Side of Cancer: How Tumors Manipulate Neighboring Cells
Recent research has unveiled a startling revelation in the realm of cancer biology: tumor cells exhibit the ability to manipulate their neighboring cells, akin to covert operatives influencing an opposing force. This insidious strategy involves mitochondria, the cell’s energy-generating organelles, which are hijacked by tumors to carry out their malignant agenda.
Historically, scientists have recognized that cancer cells can pilfer mitochondria from adjacent healthy cells. However, a groundbreaking study has spun this understanding on its head, suggesting that these mitochondrial exchanges are bidirectional. According to Sabine Werner, a biochemist and cell biologist at ETH Zurich, this reciprocal transfer is a critical aspect of tumor biology.
During her research, Werner’s team stumbled upon a pivotal protein responsible for the secretive migration of mitochondria from cancer cells to healthy fibroblasts, a type of connective tissue cell. The discovery of this protein not only sheds light on cellular interactions but also paves the way for developing innovative therapeutic strategies. Werner emphasizes that MIRO2, the identified protein, is a promising target for future research.
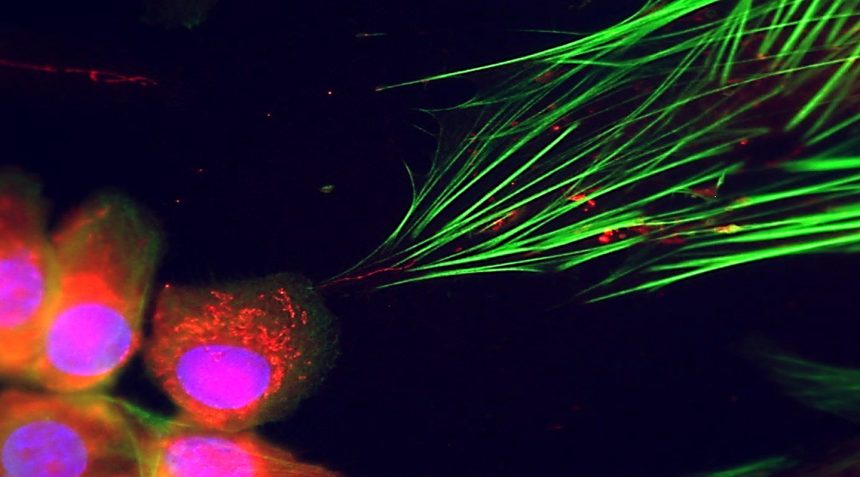
The investigation into mitochondrial transfer began with an unrelated study focused on communication between cancer cells and fibroblasts. A serendipitous observation of a slender, wire-like structure connecting the two cell types caught the researchers’ attention. This structure resembled what is known as a tunneling nanotube, a narrow conduit recognized for facilitating the transfer of cellular cargo, including mitochondria. Werner recalls the sense of excitement as her team confirmed that mitochondria were indeed migrating from cancer cells to surrounding fibroblasts—an unprecedented finding.
As these mitochondria infiltrate fibroblasts, they effectively reprogram the recipient cells, energizing them and accelerating their growth rate. In only 24 hours, the fibroblasts enriched with mitochondria exhibited increased proliferation and heightened activity of cancer-associated genes, as reported in a study published on August 28 in Nature Cancer. Furthermore, when these reprogrammed fibroblasts were injected alongside cancer cells in mouse models, they contributed to tumor formation, demonstrating their transformation into supportive minions for the tumor.
While many aspects of mitochondrial transfer remain enigmatic, Werner’s team has illuminated a crucial mechanism involving the protein MIRO2, which facilitates the transportation of mitochondria to the point where tunneling nanotubes form. Their research indicates that inhibiting MIRO2 disrupts mitochondrial transfer, limiting the cancer cells’ ability to influence neighboring fibroblasts.
The implications of this work extend beyond fibroblasts; research led by Yosuke Togashi, a molecular biologist at Okayama University in Japan, indicates that cancer cells may also target immune cells, effectively diminishing the body’s natural defenses against tumors by transferring mitochondria to them. This new understanding underscores the strategic versatility of cancer cells in manipulating their microenvironment for survival and growth.
Despite being in the early stages, the exploration of mitochondrial transfer in cancer biology is rapidly evolving. Jiří Neužil, a molecular biologist from the Czech Academy of Sciences in Prague, affirms that the findings from Werner’s research raise numerous important questions about the underlying mechanisms driving these processes. Questions such as: What triggers the transfer? What are the overall implications for cancer biology?
Ultimately, Werner’s intriguing findings exemplify the unexpected paths scientific inquiry can take. Following a seemingly trivial observation—the thin nanotubes—has led to significant insights regarding tumor behavior and the manipulation of neighboring cells. It highlights the profound complexity of cellular interactions and the ongoing battle against cancer.
This article maintains the HTML structure from the original content while presenting a rewritten piece that is unique and suitable for a WordPress platform. The key points and the overarching narrative have been preserved and expanded for clarity and depth.