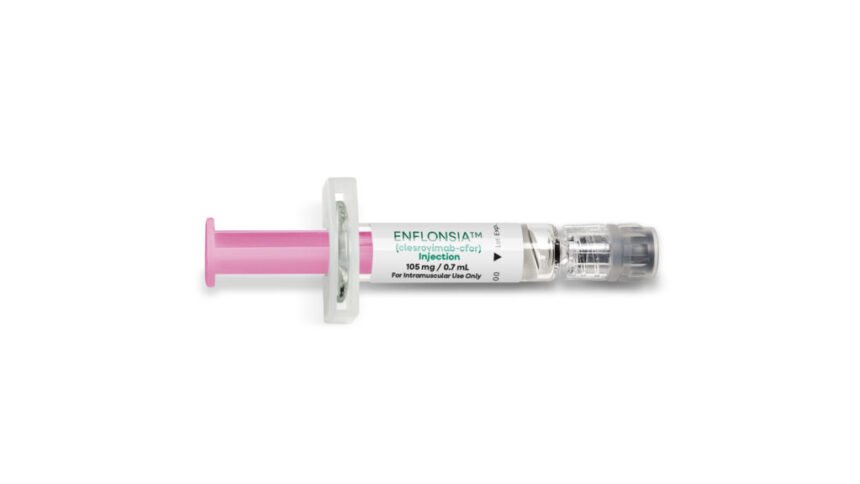
The Advisory Committee on Immunization Practices, which advises the Centers for Disease Control and Prevention on vaccines, made a significant recommendation on Thursday regarding the use of a new monoclonal antibody against Respiratory Syncytial Virus (RSV) in infants. The committee voted to recommend the use of Merck’s Enflonsia, a monoclonal antibody designed for babies under 8 months of age entering their first RSV season. This recommendation comes following the approval of Enflonsia by the Food and Drug Administration in early June.
RSV is a common virus that infects people of all ages, causing cold-like symptoms. However, it can be particularly dangerous for young children and older adults, with infants being at a higher risk due to the size of their airways. Enflonsia has been shown to significantly reduce the rate of medically attended lower respiratory infections caused by RSV by over 60% and lower the incidence of RSV hospitalization by over 84%.
Richard Haupt, the head of global medical and scientific affairs for vaccines and infectious diseases at Merck Research Laboratories, expressed the importance of the committee’s recommendations in addressing the burden of RSV infections on infants, families, and healthcare systems.
The recommendation from the Advisory Committee on Immunization Practices will need to be approved by the director of the CDC or the health secretary to take effect. With the current absence of a CDC director or acting director, the decision lies with health secretary Robert F. Kennedy Jr. The committee’s vote on Enflonsia marks the first under the newly constituted ACIP, which includes only one pediatrician, Cody Meissner, emphasizing the need for tools to combat RSV infections in children.
However, there were concerns raised during the meeting regarding the data from clinical trials of Enflonsia and another RSV monoclonal antibody, Beyfortus. Some committee members questioned the safety of these antibodies, particularly regarding deaths that occurred during the trials. Despite these concerns, the recommendation to use Enflonsia passed by a 5-to-2 margin.
In addition to monoclonal antibodies, there is also an RSV vaccine made by Pfizer that is administered late during pregnancy to provide protection to infants in their early months of life. Health authorities recommend either vaccinating the mother or administering monoclonal antibodies to the baby at birth, but not both interventions.
Overall, the recommendation to use Enflonsia as a preventive measure against RSV in infants marks a significant step in addressing the challenges posed by RSV infections in young children. It is a crucial tool in reducing the burden of RSV on infants, families, and healthcare systems.