A Groundbreaking Experiment: Creating a Microscopic Stirling Engine to Explore Thermodynamics
In a groundbreaking experiment, physicists have developed a tiny, particle-sized engine that operates at temperatures comparable to the core of the Sun. This innovative approach offers a unique opportunity to delve into the intricate realm of thermodynamics at the smallest scale.
By suspending a single silica particle in a vacuum and subjecting it to synthetic temperatures exceeding 10 million kelvin, researchers have successfully constructed a microscopic Stirling heat engine. While the primary goal was not to power a miniature device, this experiment provides valuable insights into the fundamental principles of heat and energy.
Moreover, the study sheds light on the complex microscopic processes that occur within biological systems, offering potential applications in understanding phenomena such as protein folding and mass transport.
Exploring High Temperatures and Position-Dependent Diffusion
The research, led by physicist Molly Message from King’s College London, demonstrates the promising capabilities of this experimental platform in simulating high temperatures and position-dependent diffusion. This phenomenon plays a crucial role in various biological processes, making it a valuable tool for studying dynamics at a molecular level.
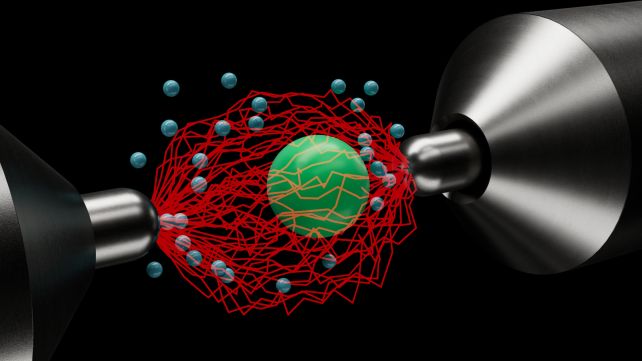
At the core of the Stirling engine concept is the cyclic process of heating and cooling a sealed fluid or gas to generate mechanical energy. In this miniature version, a silica particle measuring just 4.82 micrometers in diameter is levitated within an electric trap, enabling controlled movement without escape.
By applying electric noise to simulate temperatures up to 13 million kelvin, significantly surpassing the surface temperature of the Sun, researchers could observe the particle’s behavior under extreme conditions. This setup allows for a detailed investigation of thermodynamic processes beyond conventional limits.
Unveiling Microscopic Dynamics and Position-Dependent Diffusion
Despite the apparent violations of thermodynamic laws, such as fluctuations in heat exchange and temporary efficiency exceeding 100 percent, the system ultimately conforms to the principles of thermodynamics when averaged over time.
One of the most intriguing findings is the non-random movement of the particle within the trap, indicating position-dependent diffusion influenced by varying temperature gradients. This phenomenon mirrors the behavior of particles in biological environments, offering valuable insights into drug transport and cellular interactions.
Looking ahead, the researchers aim to further explore the intricate physics governing motion and energy at the microscopic scale, pushing the boundaries of equilibrium to uncover new insights into fundamental processes.
The findings of this study have been published in Physical Review Letters.