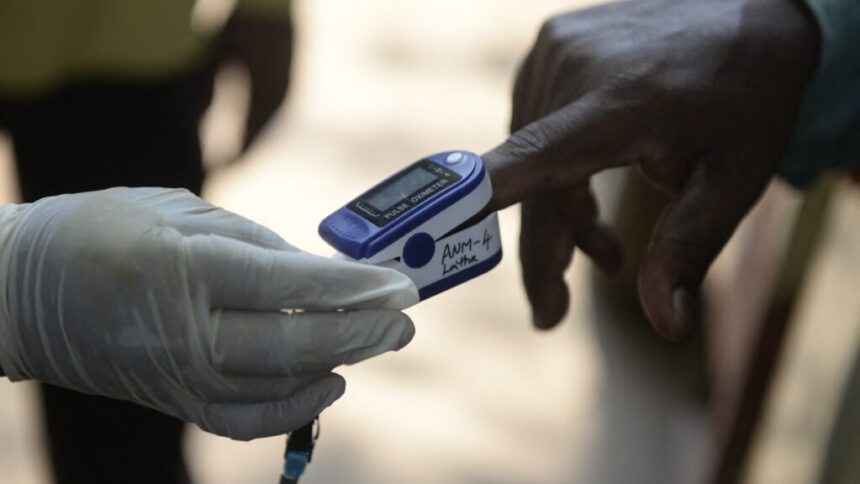
“If Medicare says, ‘We’re going to pay more for the less biased device,’ that could be a really powerful incentive for manufacturers to actually make the change.”
Ultimately, the study authors and editorial writers argue, the issue of bias in pulse oximeters is a matter of health equity and should be treated as such. Ensuring that medical devices work well on all skin tones is a crucial step in addressing health disparities and providing quality care to all patients.
As the FDA works to release new guidance and manufacturers continue to develop more equitable devices, it is clear that more needs to be done to address this long-standing issue. The health and well-being of patients, particularly those with darker skin, depend on the accuracy and reliability of medical devices like pulse oximeters. It is time for action to ensure that these devices work effectively for all individuals, regardless of their skin tone.
In order to achieve rapid adoption of newer medical devices, economic incentives play a crucial role. Without these incentives, it may be difficult to persuade healthcare providers and facilities to invest in the latest technologies. Dr. Shachar, a medical professional, expressed concerns about the effectiveness of new FDA guidance in improving the safety of devices already in use. She highlighted the need for additional measures to address the issue of faulty devices that are currently on the market.
One potential solution being discussed is the use of Section 1557 of the Affordable Care Act, which prohibits discrimination in healthcare settings. This provision could empower patients who have been harmed by faulty devices to take legal action against medical providers. A recent study revealed that a staggering 80,000 missed measurements of hypoxemia occurred in the VA system in a single year due to the use of flawed devices. It is evident that the implementation of better devices is essential to prevent such errors from recurring.
Dr. Shachar emphasized the importance of developing and introducing newer devices that do not pose the same risks as their predecessors. Once these alternatives are available, serious consideration should be given to removing older devices from circulation. This proactive approach is essential to ensure patient safety and prevent further instances of harm caused by faulty medical devices.
In conclusion, addressing the issue of outdated and faulty medical devices requires a multi-faceted approach. Economic incentives, regulatory measures, and legal safeguards must all work together to facilitate the rapid adoption of newer and safer technologies. By prioritizing patient safety and investing in innovative solutions, the healthcare industry can mitigate the risks associated with outdated medical devices and improve overall quality of care.