Investigation Launched into Weight-Loss Drugs Following Reports of Pancreas Problems
Weight-loss drugs like Ozempic and Zepbound have been praised for their effectiveness in combating obesity, but recent reports of potential side effects have raised concerns among UK health regulators.
According to the BBC, hundreds of individuals have reported severe pancreas problems after using these medications, prompting the UK government to launch an investigation into the matter. Officials are particularly interested in exploring whether genetic factors may play a role in predisposing certain individuals to such adverse reactions.
It’s important to approach this news with caution and perspective. While there is no definitive evidence linking these drugs to pancreas damage, it is crucial to highlight that these medications are safe for use when prescribed and monitored by a healthcare professional.
Doctor oversight is essential when it comes to using these drugs, as obtaining them through unofficial channels can pose serious risks. Additionally, not everyone is a suitable candidate for these medications, underscoring the importance of proper medical supervision.
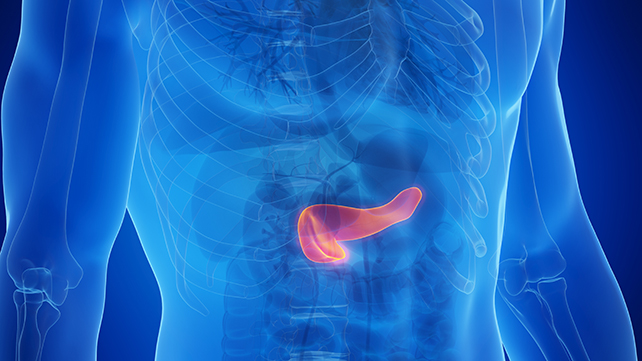
The Medicines and Healthcare products Regulatory Agency, in collaboration with Genomics England, is spearheading the investigation into these weight-loss drugs, collectively known as GPL-1 receptor agonists. These drugs target cells in the body that are typically activated by the natural GLP-1 hormone, which regulates blood sugar and appetite.
These medications are divided into two categories based on their active ingredients: those containing semaglutide (such as Ozempic and Wegovy) and those containing tirzepatide (including Mounjaro and Zepbound). The latter type also targets glucose-dependent insulinotropic polypeptide (GIP) receptors in addition to GLP-1 receptors for a more potent effect.
Recent data has revealed nearly 400 cases of acute pancreatitis associated with the use of GLP-1 drugs like Mounjaro, Wegovy, Ozempic, and liraglutide, with a significant portion linked to the tirzepatide-based drug Mounjaro.
Individuals in the UK who have experienced adverse reactions to these weight-loss and diabetes medications are encouraged to report their details on the official Yellow Card website for further investigation and potential participation in a study on GLP-1 drugs and pancreatic issues.
As with any medication, the effects of these drugs must be considered in conjunction with other factors such as existing health conditions, genetics, age, and gender. While these medications offer significant benefits, it’s essential to be aware of potential risks and seek medical guidance when using them.
“GLP-1 medicines like Ozempic and Wegovy have shown promise, but like all medications, they carry a risk of serious side effects,” stated geneticist Matt Brown from Genomics England. “We believe that genetic factors may play a role in minimizing these adverse reactions.”





