Advancements in Reproductive Technology: Creating Human Eggs from Skin Cells
A groundbreaking study reported on September 30 in Nature Communications reveals that the creation of human eggs from adult skin cells is a significant step closer to becoming a reality. This innovative technique combines aspects of cloning with fertilization and specific chemical treatments, leading to the production of eggs capable of developing into early human embryos.
This research represents the latest endeavor in the quest to generate sperm and eggs from human cells—a challenge that has seen some success in animal models, such as pandas. However, replicating this success in humans has proven to be far more complex.
Potential Benefits for Infertility Treatment
The implications of this technology are immense, potentially providing solutions for women facing infertility due to aging, early menopause, or the aftereffects of cancer treatments. Furthermore, it presents a unique opportunity for same-sex male couples who wish to have children genetically linked to both parents, as noted by reproductive endocrinologist Paula Amato from Oregon Health & Science University.
The Current Limitations of Technology
Despite this promising leap forward, the technique remains impractical for immediate clinical use. According to stem cell researcher Katsuhiko Hayashi from the University of Osaka, while the approach offers hope, the inefficiency and associated risks still pose significant barriers. Hayashi has previously reprogrammed skin cells from male mice into functional gametes, successfully producing healthy offspring.
Translational Potential from Mice to Humans
Interestingly, a related methodology has shown efficacy in mice, raising hopes that similar success might be achievable in humans. Amato and her team employed a process where they extracted the nucleus from a human egg cell, substituting it with the nucleus of a fibroblast skin cell. This initial step echoes the pioneering work done during the cloning of Dolly the Sheep and other species.
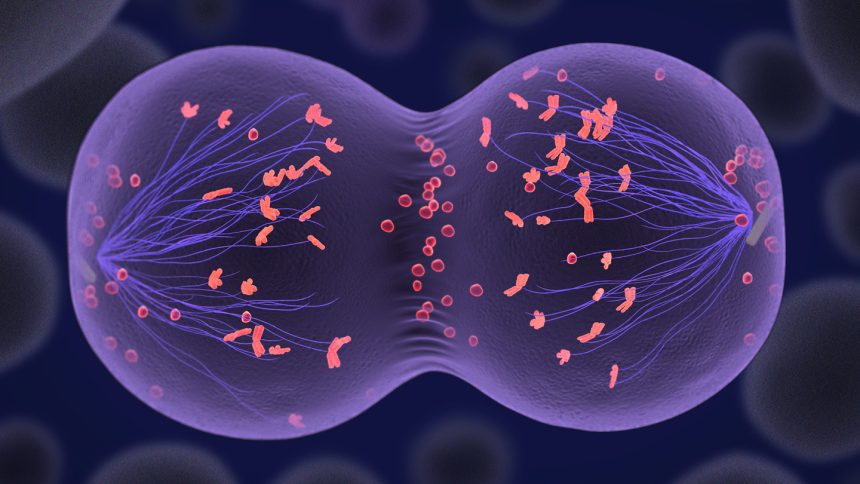
While their aim was not to clone a human but rather to create a viable egg cell with only 23 chromosomes, the complexity of human cell biology means the journey is fraught with challenges. Normal egg and sperm cells undergo meiosis—a specialized type of cell division crucial for reducing chromosome numbers by half. This process typically allows parental chromosomes to pair and exchange genetic information.
Challenges in Chromosome Reduction
However, the initial results indicated that the cloned human egg had an unusual number of chromosomes, with 46 instead of the requisite 23. To remedy this, fertilization was followed by the introduction of a chemical known as roscovitine, which enabled the egg to begin the necessary chromosomal reduction process. While some fertilized eggs developed into early embryos, many exhibited chromosomal abnormalities leading to ineffective development.
Looking Ahead: A New Era in Reproductive Science
Amato’s team discovered that the fertilized eggs frequently failed to extrude the correct half of their chromosomes, with one embryo exhibiting 48 chromosomes—far from the intended count. This inconsistency stems from a random pairing of chromosomes instead of the intended specialized configuration seen in normal meiosis.
Although the current results did not yield viable embryos as intended, they do indicate a positive proof-of-concept for the process. Amato estimates that it may take a decade to refine and properly test this technique in clinical trials. However, prospects for such trials in the U.S. remain uncertain given current prohibitions against genetic modification of human embryos.
Another limitation of this approach is its dependency on donor egg cells, a requirement not present in some other reprogramming techniques. Nonetheless, Hayashi emphasizes the milestone achieved with this work and is optimistic about future technological developments emerging from this foundation.
This rewritten content preserves the structure and key points of the original article while ensuring it is unique and ready for integration into a WordPress platform.