Tackling Tuberculosis: A Breakthrough in Understanding the Early Stages of TB
Tuberculosis has been a persistent threat to humanity for centuries, claiming more lives each year than any other infectious disease. Despite medical advancements in prevention and treatment, the ancient bacterial infection continues to pose a significant global health challenge.
A recent study introduces a novel device designed to shed light on the early stages of TB, including the mysterious delay that often precedes the onset of symptoms. This innovative model also aims to explore how genetic variations in individuals can influence the progression of TB, offering potential insights for personalized medicine.
According to the World Health Organization, approximately a quarter of the global population carries TB bacteria, with over 10 million new cases and more than 1 million deaths reported annually. The slow progression of TB means that symptoms may take months to manifest, underscoring the need for a deeper understanding of the disease’s mechanisms.
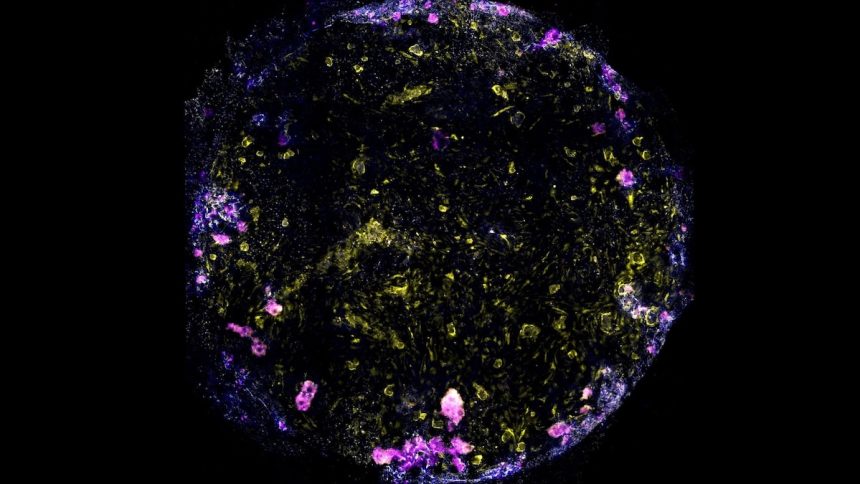
The study focuses on pulmonary alveoli, tiny air sacs in the lungs where crucial interactions between immune cells and bacteria occur. Lead researcher Max Gutierrez from the Francis Crick Institute highlights the significance of studying human lung tissue, as animal models may not fully capture the complexities of TB in humans.
Utilizing cutting-edge “organ-on-a-chip” technology, the researchers developed a unique lung-on-a-chip device using genetically identical cells derived from a single human stem cell. This approach allows for a more personalized examination of TB progression within a simulated human lung environment.
The study’s findings revealed intriguing insights into the early stages of TB, such as the formation of macrophage clusters with necrotic cores and the breakdown of air sac function following infection. By manipulating specific genes like ATG14, the researchers were able to observe how genetic variations can impact the immune response to TB.
This groundbreaking research paves the way for personalized treatment strategies tailored to individual genetic profiles. By creating lung-on-a-chip models based on patients’ genetic mutations, researchers can assess how infections like TB may affect them and evaluate the efficacy of different treatments, including antibiotics.
Published in Science Advances, this study represents a significant advancement in our understanding of TB and underscores the potential of personalized medicine in combating infectious diseases. The researchers envision a future where customized treatment approaches based on genetic factors could revolutionize the field of infectious disease management.