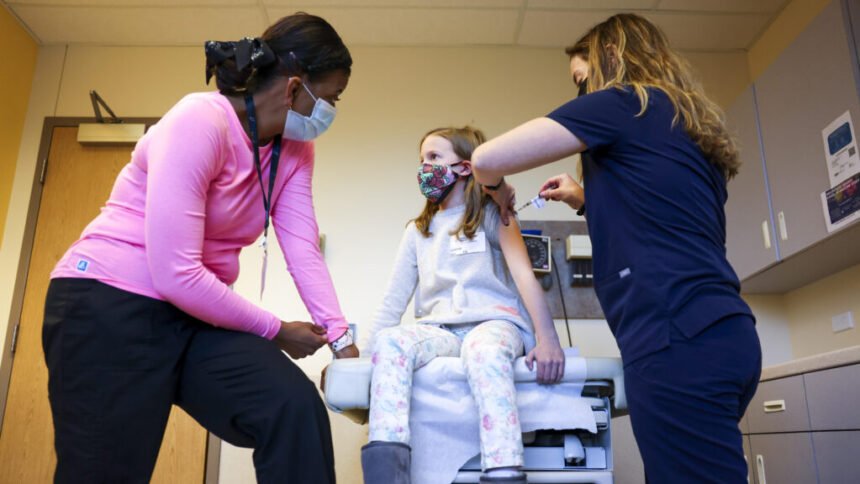
The announcement of a new framework for Covid-19 vaccines by Food and Drug Administration (FDA) leaders has sparked discussion and confusion among physicians regarding its implications, particularly for children. The framework suggests that the FDA will no longer approve new Covid vaccines for healthy individuals under 65, including babies, without data from new randomized clinical trials showing their benefit.
The plan, outlined in a commentary in the New England Journal of Medicine and discussed in a livestream on YouTube, focused on the need for more data supporting the ongoing use of annual booster shots. However, it also alluded to a lack of evidence for children as young as 6 months, who currently receive a two- or three-dose “primary” series of vaccinations.
Physicians and infectious disease specialists were left with numerous questions following the announcement. Concerns were raised about how the framework would impact childhood vaccinations, the duration of existing shots’ validity, and the eligibility criteria for high-risk individuals. Pediatricians expressed alarm at the possibility of Covid shots becoming unavailable for children, emphasizing the importance of safe and effective vaccines for this vulnerable population.
The commentary titled “An Evidence-Based Approach to Covid-19 Vaccination” drew criticism from experts like James Campbell, who argued that decisions on vaccines should involve comprehensive evidence review and debate among working groups rather than being made by a select few. The commentary’s tone and implications were deemed concerning by many in the medical community.
FDA Commissioner Marty Makary and vaccine regulator Vinay Prasad defended the framework during the livestream, with Prasad expressing skepticism about the necessity of Covid vaccines for children. He cited statistics showing that young children faced a lower risk of severe disease compared to older age groups, questioning the need for widespread vaccination in this population.
Despite the controversy surrounding the new framework, some experts like Amesh Adalja supported the idea of including Covid vaccines in routine childhood vaccinations. Adalja emphasized the importance of early vaccination to ensure children have immunity, especially considering the potential risks associated with the virus.
Emergency physician Jeremy Faust underscored the need to prioritize infants aged 6 months and older, who are considered high-risk and should receive the vaccine to prevent severe illness. Faust highlighted the importance of erring on the side of caution when assessing the cost-benefit scenarios for infants, emphasizing the need to prioritize their health and safety.
As the medical community grapples with the implications of the new framework, organizations like the American Academy of Pediatrics are working to gather more information and communicate any changes to their members and the public. The debate around Covid-19 vaccines for children continues, with experts advocating for a balanced approach that prioritizes safety and efficacy. As the Covid-19 pandemic continues to evolve, many vaccine experts are reevaluating the current approach to vaccination. With a significant portion of the adult population already having some level of immunity to the virus through vaccines or prior infections, there is a growing consensus that a more targeted approach may be more effective moving forward.
One of the key concerns raised by experts is the impact of new restrictions on vulnerable populations, such as those who live with or interact with high-risk individuals. While some argue that vaccinating individuals who interact with high-risk individuals could potentially save lives, others believe that vaccines should be prioritized for those at the highest risk of severe illness or complications.
In line with this thinking, some experts suggest aligning Covid vaccine policy with that of other vaccines, such as the RSV vaccine, which is primarily offered to high-risk individuals. By focusing on those most vulnerable to severe Covid-19 outcomes, resources can be allocated more effectively to protect those who need it most.
Healthcare workers are another group that has been at the forefront of the vaccination debate. While some argue that healthcare workers should be prioritized for vaccines to reduce the risk of transmission and infection, others point to low uptake rates among healthcare workers as a reason to reconsider blanket vaccination recommendations.
However, the shift towards a more targeted approach to vaccination could have unintended consequences for underserved communities, particularly Black, Hispanic, and Native American populations that have been disproportionately affected by the pandemic. These communities already face barriers to accessing healthcare and vaccines, and restricting access to Covid vaccines could further widen existing health disparities.
Furthermore, the issue of long Covid, a condition characterized by persistent symptoms following a Covid-19 infection, has been largely overlooked in discussions around vaccine eligibility. Some individuals with long Covid argue that evidence showing the benefits of vaccination in reducing the risk of long-lasting symptoms should be considered in vaccine policy decisions.
Overall, the debate around Covid vaccine recommendations highlights the need for a nuanced and evidence-based approach to vaccination policy. While concerns about vaccine hesitancy and misinformation are valid, experts emphasize the importance of prioritizing those most at risk and addressing health disparities to effectively combat the ongoing pandemic.