Weight loss drugs like Wegovy (semaglutide) and Mounjaro (tirzepatide) are gaining popularity among individuals looking to shed excess weight. However, concerns have been raised about the impact of these medications on unintended pregnancies, leading the UK’s medicines regulator to issue guidance for women of reproductive age.
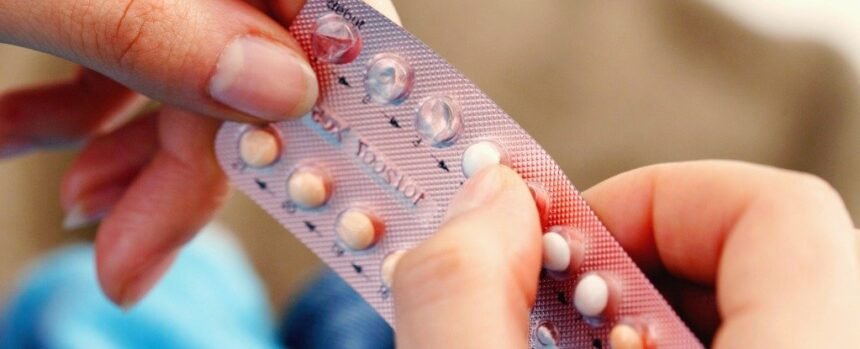
The Medicines and Healthcare products Regulatory Agency (MHRA) received 40 reports of unintended pregnancies in women using weight loss drugs. Of particular concern is the effect these medications may have on the effectiveness of oral contraceptives.
These weight loss injections work by mimicking the hormone GLP-1, which is released from the gut after eating and helps suppress appetite. Tirzepatide also acts on another hormone system called GIP, which also aids in appetite suppression.
The mechanism by which these drugs affect appetite is complex. They inhibit hunger-associated brain regions, reducing the increase in appetite that often occurs during weight loss. Additionally, GLP-1 drugs slow down stomach emptying, further contributing to appetite suppression.
Research on the interaction between GLP-1 drugs and oral contraceptives is limited. However, a recent study found that tirzepatide reduced the absorption of ethinylestradiol (a key component of oral contraceptives) by 20% and delayed its absorption into the bloodstream by two to four hours. This reduced absorbency can compromise the contraceptive effects of the pill.
Side effects like vomiting and diarrhea, common with GLP-1 drugs, can also interfere with the absorption of oral medications, including contraceptives. These side effects may lead to the expulsion of the drugs from the body before proper absorption, reducing their effectiveness.
Weight loss in general can impact fertility, and the use of GLP-1 drugs may increase fertility, potentially increasing the likelihood of unintended pregnancies. While non-oral contraceptives are unlikely to be affected by these weight loss drugs, women using oral contraceptives are advised to use additional forms of contraception for four weeks after starting semaglutide or tirzepatide.
In case of pregnancy while using these medications, women are urged to consult their healthcare provider for alternative options due to the lack of safety data during pregnancy. It is crucial for women to be aware of the potential effects of weight loss drugs on contraceptive efficacy and take necessary precautions to prevent unintended pregnancies.