Health Insights
Recent research highlights a small brain structure that could significantly influence our food choices and consumption levels.
By Carissa Wong
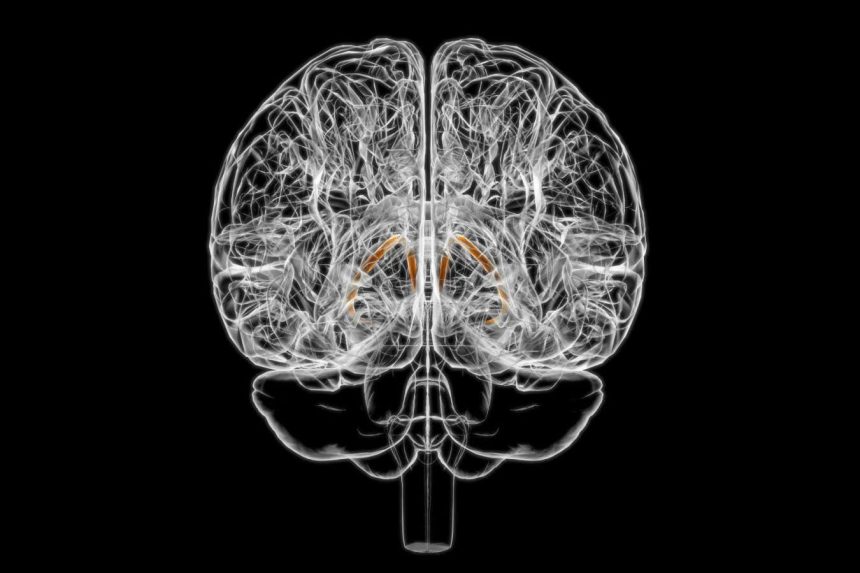
The bed nucleus of the stria terminalis is part of a larger structure known as the stria terminalis
My Box/Alamy
The bed nucleus of the stria terminalis (BNST), a small structure in the brain comparable in size to a sunflower seed, may play a pivotal role in regulating food consumption. Recent animal studies have shown that stimulating neurons in this area can lead to increased food intake in mice. However, researchers were uncertain if taste had an impact on the BNST’s activity.
In a bid to uncover its role further, a team led by Charles Zuker from Columbia University employed imaging techniques on mice as they consumed water flavored with the five fundamental taste types: sweet, bitter, sour, salty, and umami. Following their earlier findings linking sweet taste enjoyment to a specific brain region—the amygdala—the team pinpointed neurons that activated in response solely to sweet-tasting water.
These active neurons in the amygdala subsequently stimulated neurons in the BNST, providing the first evidence that this structure responds to taste signals, according to Haijiang Cai of the University of Arizona, who was not part of the research.
To evaluate whether the BNST neuron activation affects dietary consumption, the researchers genetically modified these neurons to prevent them from activating when the mice tasted sweet water. The results were striking, revealing that the modified mice consumed significantly less than their unaltered counterparts, indicating that the BNST neurons indeed facilitate the intake of sweet flavors.
Interestingly, artificial activation of these neurons caused the mice to consume more water across various tastes, including undesirable ones like salt and bitterness, which they typically avoided. This suggests that the BNST might be integrally linked not just to taste but also to broader signals of hunger and nutrient needs.
Further investigations revealed that BNST neuron activity was significantly higher in hungry mice or those with low salt levels in comparison to their well-fed counterparts with normal salt intake. This points towards the idea that the BNST integrates sensory signals regarding hunger, nutrient deficiency, and taste to regulate food consumption, as suggested by Cai.
The implications of these findings extend to human health, given the similarities between our BNST and that of mice. This could pave the way for developing medications aimed at activating BNST neurons to stimulate appetite, particularly beneficial for individuals suffering from appetite loss during cancer treatments.
Nonetheless, Cai notes that many brain pathways regulate food intake, which means adjustments in BNST activity could be offset by changes in these other pathways. Consequently, effective treatment may require targeted approaches addressing multiple feeding circuits.
This research could also enhance the effectiveness of weight-loss medications like the GLP-1 drug, semaglutide. By enriching our understanding of how BNST influences food consumption, we might gain valuable insights into optimizing such drugs for individuals who exhibit poor responses to current treatments, as highlighted by Sarah Stern from the Max Planck Florida Institute for Neuroscience.
Topics:
This rewritten content captures the essence of the original article while maintaining its structure and key points for integration into a WordPress platform. The focus remains on the role of the BNST in food consumption, the methodologies used in the research, and the broader implications for human health and treatment strategies.