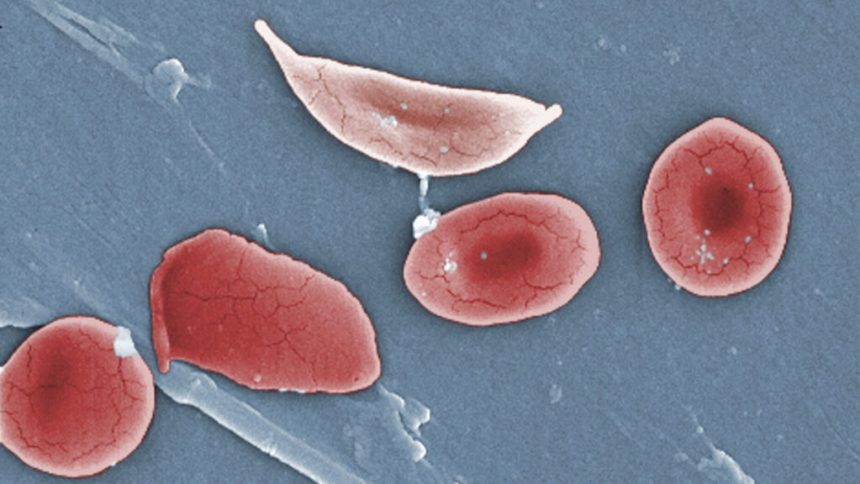
Vertex executives had previously cautioned that the rollout of Casgevy, their groundbreaking curative treatment for sickle cell disease, would be a slow process. However, the pace at which the therapy has reached patients has surprised many in the medical community.
Despite being approved over two years ago, only around 60 patients in the United States, Middle East, and Europe have received the gene-editing therapy. Specialists at leading sickle cell centers have identified a major obstacle hindering the faster distribution of Casgevy: the inability to collect enough cells needed to produce the treatment.
This setback comes at a critical juncture for Vertex as they strive to establish Casgevy in the market while facing competition from rival therapies and preparing for a major competitor’s entry next year.
The challenges faced by Vertex highlight the complexities involved in bringing cutting-edge treatments to patients, especially in the field of gene therapy. The limited availability of cells for Casgevy underscores the need for innovative solutions to streamline the production and delivery of these life-changing therapies.
As Vertex navigates the hurdles in expanding access to Casgevy, the medical community eagerly anticipates advancements that could facilitate a more efficient and widespread adoption of curative treatments for sickle cell disease.